Breaking News | Salus Pro Receives Chinese NMPA Regulatory Approval!
On January 20, 2025, Salus Pro received Class III medical device registration approval from China's National Medical Products Administration (NMPA) (Registration No. 20253220191). This means that Salus Pro is now legally approved under China's medical regulatory system to sequence human DNA, RNA, and microbial DNA/RNA derived from the human body. It is also the first sequencer in the Chinese market to receive approval for comprehensive clinical applications across all use cases in China.

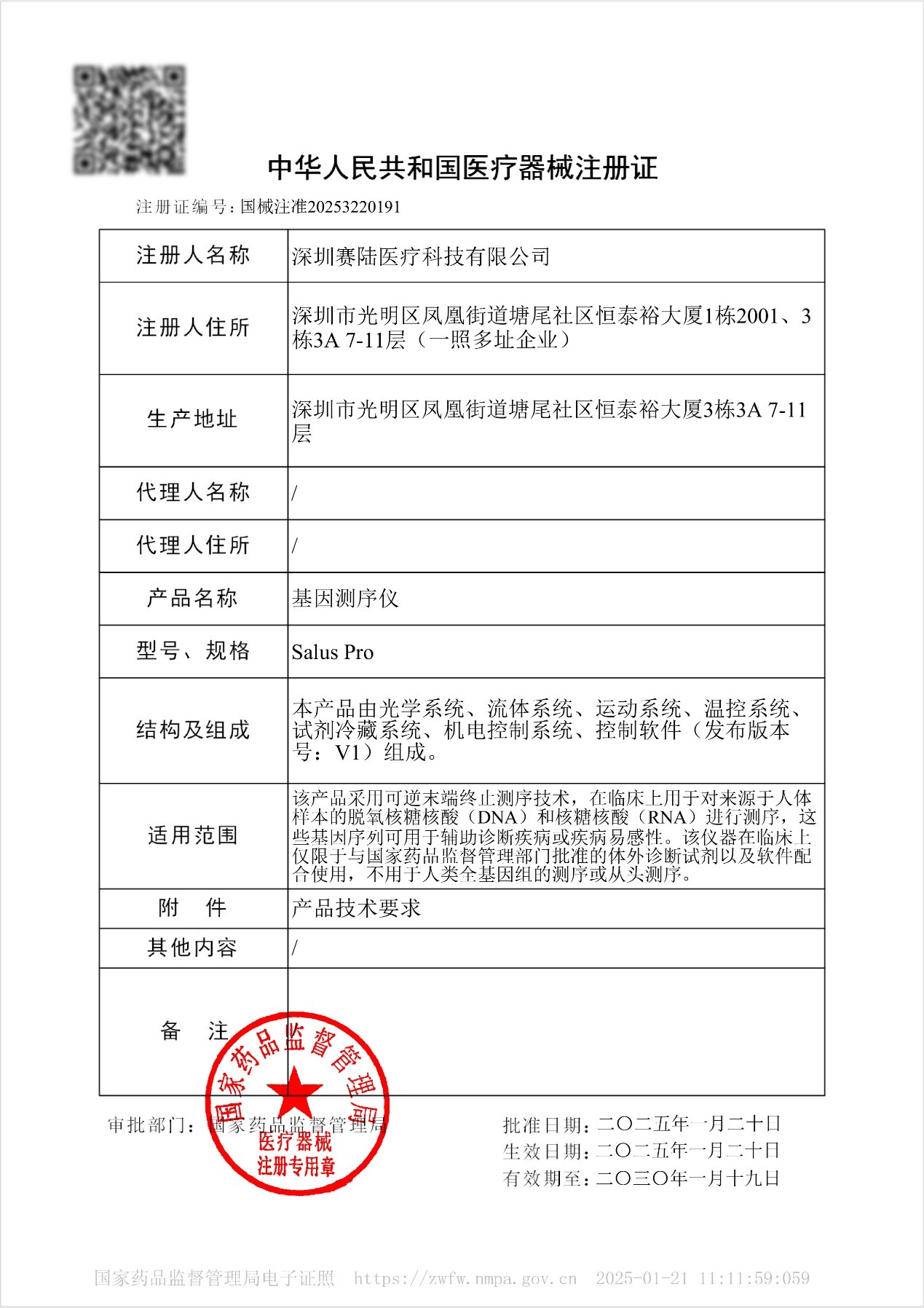
Salus Pro
Salus Pro offers multiple sequencing reagent sets to support various sample sizes or different applications.

The Salus Pro IVD Device has two sequencing units, supporting simultaneously or rolling up sequencing. And it offers multiple chip size (80M, 150M, 250M, and 500M) along with various read lengths (SE50, SE75, SE100, PE100, PE150), catering to address diverse needs in clinical and research applications.
The NMPA approval of the Salus Pro IVD Device marks a milestone for the company development of Salus. We look forward to collaborating with more partners across various fields to develop innovative solutions in reproductive health, oncology, infection control, and general health, meeting the diverse sequencing needs of both clinical and research applications.


